AMSBIO has published an interview with researchers at Yamaguchi University Graduate School of Medicine, Department of Gastroenterology and Hepatology, Japan, that covers their groundbreaking work to develop cell regeneration therapies for liver cirrhosis.
The interview covers the involvement of the Graduate School of Medicine in the development of StemFit® for Mesenchymal Stem Cell (MSC), a new chemically defined medium which minimizes the risk of hazardous animal derived ingredients, and how the product has assisted cutting-edge liver regeneration therapy research and efforts towards upcoming clinical trials.
When asked, Associate Professor Taro Takami commented “By culturing MSCs using StemFit for MSC, we were able to efficiently grow our cells to a clinical standard and maintain important MSC functions at a high level (lower levels of inflammatory cytokine expression, higher levels of anti-inflammatory cytokine expression and cell senescence markers). We are happy that it has become possible to culture high-quality cells by this medium so that we can proceed now to a clinical trial. We hope that our regenerative medicine products will receive regulatory approval as soon as possible and can be delivered to many patients with cirrhosis”.
StemFit® for MSC is a chemically defined and human/animal origin free medium, optimized for the isolation and culture of MSCs from bone marrow (BM-MSC), umbilical cord (UC-MSC), and adipose (ADSC) under serum-free, human platelet lysate free conditions. The fully chemically defined formulation of StemFit® for MSC minimizes lot-to-lot variation and enables highly consistent and superior cell expansion compared to serum containing media. Containing no animal or human-derived components minimizes risk of immunogenic contamination making StemFit® for MSC also extremely safe to use. Extensively tested, StemFit® for MSC offers exceptional cell expansion with high levels of marker expression and differentiation potential. For best results, AMSBIO recommend using StemFit® for MSC with iMatrix-511 as a cell culture substrate.
To read the interview in full please visit https://www.amsbio.com/news/taro-takami-interview/. For further information on StemFit® for MSC please visit https://www.amsbio.com/stemfit-for-mesenchymal-stem-cells/ or contact AMSBIO on +44-1235-828200 / +1-617-945-5033 / [email protected].
Founded in 1987, AMS Biotechnology (AMSBIO) is recognized today as a leading transatlantic company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products and services for research and development in the medical, nutrition, cosmetics and energy industries. AMSBIO has in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion and proliferation. This expertise in cell culture and the ECM allows AMSBIO to partner with clients in tailoring cell systems to enhance organoid and spheroid screening outcomes using a variety of 3D culture systems, including organ-on-a-chip microfluidics. For drug discovery research, AMSBIO offers assays, recombinant proteins and cell lines. Drawing upon a huge and comprehensive biorepository, AMSBIO is widely recognised as a leading provider of high-quality tissue specimens (including custom procurement) from both human and animal tissues. The company provides unique clinical grade products for stem cell and cell therapy applications these include high quality solutions for viral delivery (lentivirus, adenovirus and adeno-associated virus) in addition to GMP cryopreservation technology.
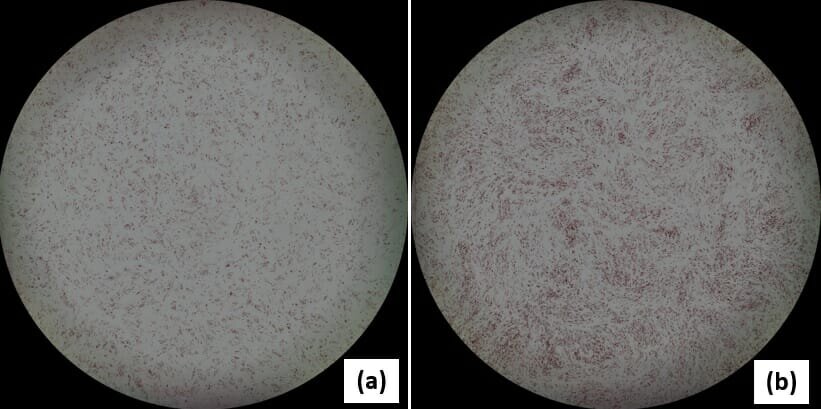
Image caption: Adipogenic differentiation:Oil-red O staining of bone marrow derived MSCs cultured with (a) standard medium (10%FBS+DMEM) or (b) StemFit For MSCs. Image courtesy of Taro Takami (Yamaguchi University Graduate School of Medicine, Japan).