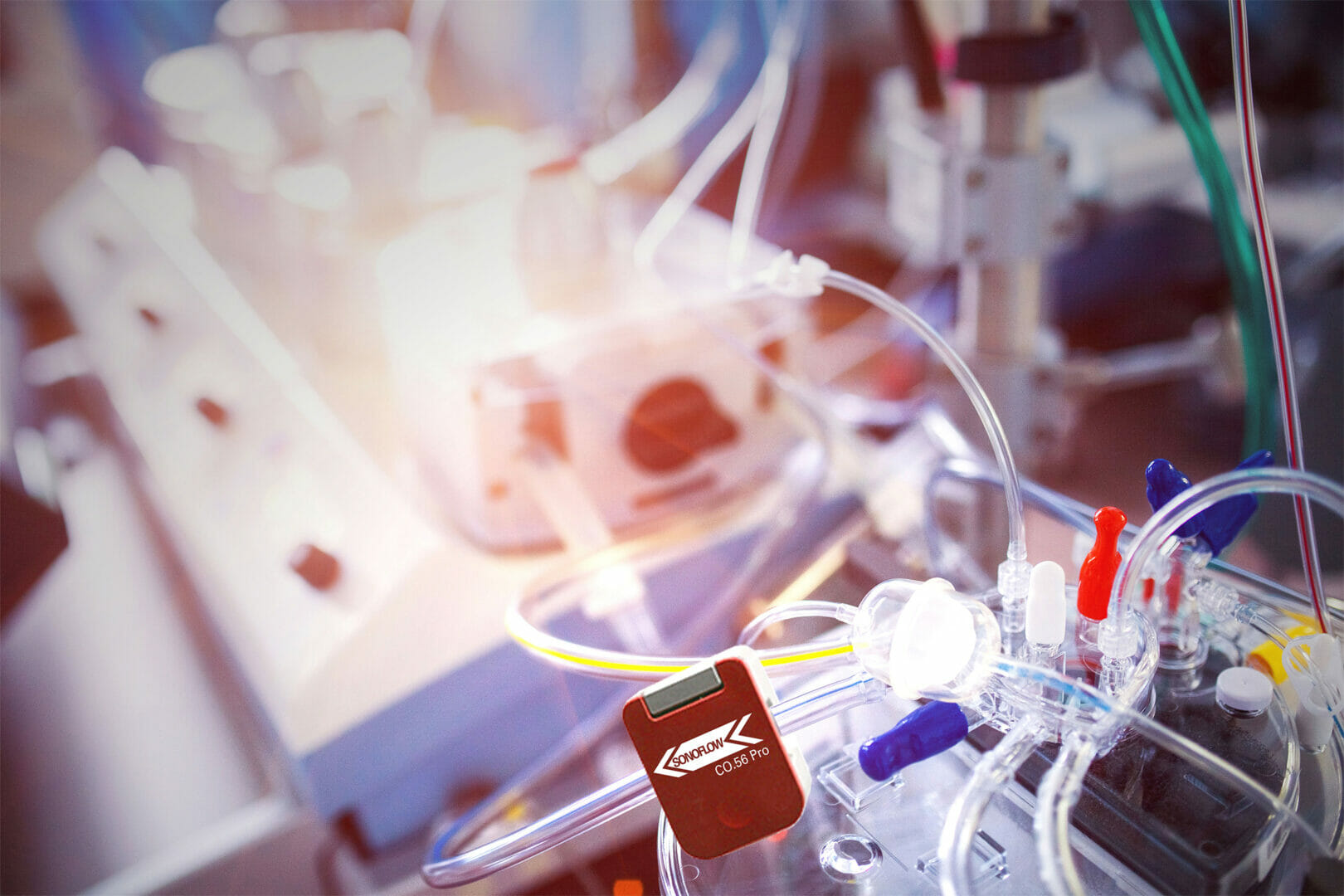
As developer and manufacturer of ultrasonic sensors and expert in the implementation of sensors for bubble detection and fluid monitoring in medical technologies, SONOTEC has made a name for itself, gaining worldwide industry reputation over decades. With the ISO 13485 certification standard, the company meets the highest requirements for a comprehensive quality management system to design and manufacture medical products. At COMPAMED 2019, SONOTEC celebrates the market launch of the new flagship sensor SONOFLOW CO.56 Pro for combined FlowBubble Measurement in medical products.
“This launch takes our SONOFLOW CO.56 success to a new level,” says International Sales Manager Lutz Schmidt, explaining the new sensor concept. The sensors are technologically leading in the global field of competition, Schmidt adds. The non-invasive flow and bubble sensor captivates through its compact design and integrated electronics, can be mounted freely suspended, and offers the option of multi-point measurement. In this way, up to twelve independent sensors, even with different channel sizes, can be operated with just one control unit. The built-in microcontroller guarantees to control all processes whose data is transferred via RS485-interface. The sensors themselves operate to measure flow rates on different tubes and shunts and simultaneously detect air bubbles accidentally caused to prevent from dangerous air embolism. Finally, the non-invasive ultrasonic sensors give feedback on the real flow compared to the theoretical flow of the pump.
As a leading sensor technology specialist in medical technology SONOTEC easily meets the latest EMC product standard IEC 60601-1-2 (Edition 4) for the SONOFLOW CO.56 Pro FlowBubble Sensor. In addition to Edition 4, the sensors meet IEC 60601-1 (Edition 3) and IEC 61157, the standard means for the reporting of the acoustic output of medical diagnostic ultrasonic equipment, and RoH’s 2011/65/EU to guarantee CE compliance.
SONOTEC with a wide-ranging product portfolio and a full service promise at the COMPOMED fair stand
Alongside the focus on combined flow- and bubble measurement, SONOTEC will be additionally presenting its full range of non-invasive sensor-engineered medtech solutions. The sensors of the SONOFLOW & SONOCHECK family are applied to fulfil tasks in flow measurement as well as air bubble & blood leak detection. Over a long period of successfully implemented projects, the German-based ultrasound specialist has gained an excellent reputation among manufacturers of dialysis and transfusion devices, heart-lung machines, blood separators, medical pumps and diagnostic systems. The intensive guidance from a very early stage in the product development process, support in verification, prototyping and its expertise in certification processes gives SONOTEC a lasting competitive edge.
“With the long-term experience and reputation for high quality products, we intend to make our sensors a touch and feel experience at our stand”, explains Marketing Manager Daniel Thieme the 2019’s exhibition philosophy. “For that reason, we will be travelling with a number of product set-ups and sensor models to Dusseldorf”, marketing colleague Frauke Beelmann adds somewhat proud.
Visit SONOTEC from 18 to 21 November 2019 at booth N08 in hall 8A at COMPAMED in Düsseldorf.